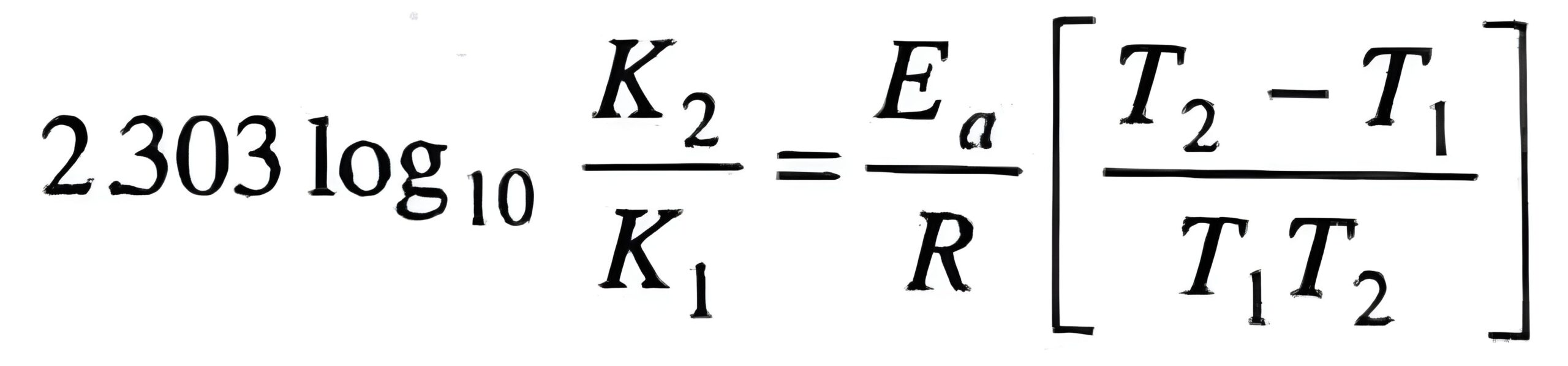Chemical Kinetics | Class 12 CBSE Board Exam 2025
Definitions, Formulas, and Predicted Questions
The chapter Chemical Kinetics in Class 12 Chemistry is crucial for CBSE Board Exams 2025. It delves into the study of reaction rates, factors affecting them, and the mechanisms by which reactions proceed. This chapter forms a fundamental part of physical chemistry and has consistently contributed significant weightage to the board exams. By analyzing previous years’ papers and the 2024-25 syllabus, we predict the most important topics and questions that are likely to be asked in the 2025 board exam. This guide provides a structured approach to understanding the chapter, with definitions, formulas, probable question types, and examples.
10 Most Important Topics for Chemical Kinetics
1. Rate of Reaction
- Definition: The change in concentration of reactants or products per unit time.
- Formula: Rate = -d[R]/dt = d[P]/dt
- Expected Question Types:
- MCQ: Define the rate of reaction.
- Very Short Answer: What is the unit of rate of reaction?
- Short Answer: Derive the formula for the rate of reaction for a given example.
- Example Question: Calculate the rate of reaction if 0.1 mol/L of reactant is consumed in 10 seconds.
Hint: Use the formula Rate = -d[R]/dt.
2. Order of Reaction
- Definition: The sum of powers of the concentration terms in the rate law equation.
- Formula: For a reaction A + B → C, Rate = k[A]m [B]n, where (m + n) is the order.
- Expected Question Types:
- MCQ: Identify the order of reaction from the rate equation.
- Short Answer: Explain the concept of order of reaction with examples.
- Competency-Based: Analyze experimental data to determine the order of a reaction.
- Example Question: For a reaction 2A → B, if the rate law is Rate = k[A]2, what is the order of reaction?
Hint: Order is the sum of the powers of concentration terms.
3. Integrated Rate Laws for Zero and First Order Reactions
- Formulas:
- Zero-Order: [R] = [R₀] – kt
- First-Order: ln[R] = ln[R₀] – kt or [R] = [R₀] e-kt
- Expected Question Types:
- MCQ: Identify the integrated rate law for a first-order reaction.
- Long Answer: Derive the integrated rate law for a first-order reaction.
- Assertion-Reason: “A zero-order reaction’s rate is independent of reactant concentration.”
- Example Question: Derive the integrated rate law for a first-order reaction and give its graphical representation.
Hint: Use the formula and show a plot of ln[R] vs. time.
4. Half-Life of a Reaction
- Definition: Time taken for the concentration of a reactant to reduce to half its initial value.
- Formula:
- First-Order Reaction: t1/2 = 0.693/k
- Zero-Order Reaction: t1/2 = [R₀]/2k
- Expected Question Types:
- MCQ: Calculate the half-life for a given first-order reaction.
- Short Answer: Compare half-lives of zero-order and first-order reactions.
- Example Question: Calculate the half-life for a first-order reaction with rate constant k = 2 × 10-3 s-1.
Hint: Use t1/2 = 0.693/k.
5. Temperature Dependence of Rate: Arrhenius Equation
- Formula: k = A e-Ea/RT
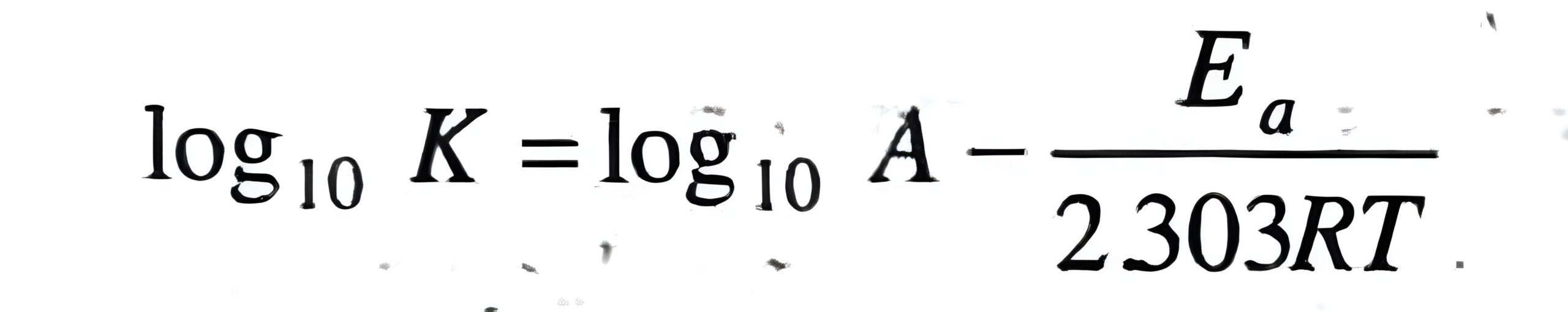
- Expected Question Types:
- MCQ: What is the effect of temperature on the rate constant?
- Short Answer: Explain the significance of Ea in the Arrhenius equation.
- Competency-Based: Use experimental data to determine activation energy.
- Example Question: The rate constant for a reaction doubles when the temperature is increased from 298 K to 308 K. Calculate the activation energy.
Hint: Use the Arrhenius equation in the formArrhenius Equation
6. Collision Theory of Reaction Rates
- Definition: Reaction occurs when reactant particles collide with sufficient energy and proper orientation.
- Expected Question Types:
- MCQ: Identify the condition for effective collision.
- Short Answer: Explain the role of activation energy in collision theory.
- Example Question: How does collision theory explain the effect of temperature on reaction rate?
Hint: Higher temperature increases the number of effective collisions.
7. Catalysis and its Effect on Reaction Rate
- Definition: A catalyst speeds up a reaction without being consumed by lowering the activation energy.
- Expected Question Types:
- MCQ: How does a catalyst affect the activation energy of a reaction?
- Assertion-Reason: “Catalysts increase the rate of both forward and reverse reactions.”
- Example Question: Discuss the role of a catalyst in a chemical reaction with the help of an energy profile diagram.
Hint: A catalyst lowers Ea, providing an alternate reaction pathway.
8. Pseudo-First-Order Reactions
- Definition: Reactions that are second-order but appear to be first-order due to one reactant being in excess.
- Expected Question Types:
- MCQ: Identify a pseudo-first-order reaction from given options.
- Short Answer: Explain why hydrolysis of esters is considered a pseudo-first-order reaction.
- Example Question: Why is the hydrolysis of an ester in the presence of water a pseudo-first-order reaction?
Hint: One reactant (water) is present in excess.
9. Activation Energy and Reaction Mechanism
- Definition: The minimum energy required to initiate a reaction.
- Expected Question Types:
- MCQ: Identify the correct definition of activation energy.
- Short Answer: What is the role of activation energy in determining the rate of reaction?
- Example Question: Discuss how activation energy influences the rate of reaction using a suitable example.
Hint: Reactions with high activation energy proceed slower.
10. Case Study-Based Questions
- Definition: Involves real-world scenarios where concepts of chemical kinetics are applied.
- Expected Question Types:
- Case Study: Analyze the factors affecting the rate of decomposition of hydrogen peroxide under different conditions.
- Example Question: In an industrial setup, the decomposition of hydrogen peroxide is catalyzed by MnO₂. Discuss how temperature and catalyst concentration affect the rate.
Hint: Use concepts of temperature dependence and catalysis.
Conclusion:
In summary, Chemical Kinetics is an essential chapter in Class 12 Chemistry, contributing significantly to the CBSE board exam. Key concepts such as the rate of reaction, order of reaction, half-life, and the temperature dependence of reaction rates play a crucial role in understanding how chemical processes occur. By mastering the formulas and derivations, and understanding the influence of factors like concentration, temperature, and catalysts, you can perform well in this section.
Important Points to Remember:
To score well in the 2025 board exams, it’s important to:
- Focus on the key formulas, especially for zero and first-order reactions.
- Be ready to solve numerical problems related to half-life, rate constants, and activation energy.
- Practice different types of questions like MCQs, assertion-reason, case studies, and competency-based questions.
- Ensure you understand graphs, especially for integrated rate laws, as they are frequently asked.
- Focus on first-order and zero-order reaction derivations.
Chemical Kinetics holds immense importance in the CBSE Class 12 Chemistry curriculum. A solid grasp of concepts such as rate of reaction, order of reaction, and the temperature dependence of rates can significantly enhance your performance in the 2025 board exams.
To enhance your preparation even further for CBSE 2025 Exam, you can also connect with us on YouTube, where we will guide you through all these topics in a comprehensive manner. To join us on YouTube, simply click the link below, and you’ve already taken your first step towards scoring better marks.
In the next post, we will discuss the key topics of the chapter ‘d & f-Block Elements.‘
Note: All the topics covered in this article are based on the “Chemical Kinetics” chapter of Class 12 CBSE Board Chemistry and have been curated after analyzing previous years question papers. This article can be helpful for students aiming for success in exams, but it’s also important to thoroughly understand the entire syllabus and practice regularly.
Thank you!
Best wishes to all students!
Class 12 Chemistry CBSE 2025 Exam: Important Topics for Objective, Competency-Based, Case Study, and Assertion-Reason Type Questions Click Here
Class 12 Chemistry CBSE 2025 Exam: 10 Most Important Topics from ‘Solutions’ Chapter Click Here
10 Most Important Topics from ‘Electrochemistry’ Chapter: Class 12 Chemistry CBSE 2025 Exam Click Here