The chapter Acids, Bases, and Salts from Class 10 Science is crucial for CBSE board exams and covers fundamental concepts that are widely applied in chemistry and everyday life. This chapter holds significant weightage in the board exams, with both conceptual and application-based questions often appearing in the papers. By analyzing previous years’ questions and current trends, this guide highlights the 10 most important topics to focus on, giving students a clear understanding of what they need to prioritize for the CBSE 2025 Board Exams. Understanding definitions, reactions, properties, and real-world applications is essential for scoring well in this chapter.
10 Most Important Topics from Acids, Bases, and Salts
(Class 10 Science)
Here’s a priority list of topics based on previous year trends, with an analysis of the types of questions likely to be asked.
1. Understanding Acids, Bases, and Salts
- Definitions:
- Acid: A substance that produces H⁺ ions in aqueous solutions (e.g., HCl, H₂SO₄).
- Base: A substance that produces OH⁻ ions in aqueous solutions (e.g., NaOH, KOH).
- Salt: A compound formed from the neutralization of an acid by a base (e.g., NaCl, K₂SO₄).
- Types of Questions: 1-mark definition-based questions, MCQs.
2. Chemical Properties of Acids and Bases
- Key Reactions:
- Acids react with metals to produce salt and hydrogen gas.
- Example: 2HCl + Zn → ZnCl2 + H2↑
- Bases react with acids (Neutralization reaction):
- Example: HCl + NaOH → NaCl + H2O
- Acids react with metals to produce salt and hydrogen gas.
- Types of Questions: 2- or 3-mark questions with reaction-based problems, reaction completion or short answer reasoning.
3. pH Scale and Importance of pH in Everyday Life
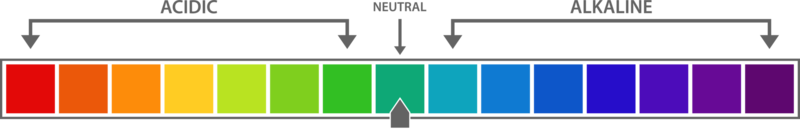
- Formula: pH = −log[H+]
- Key Points:
- Scale ranging from 0 to 14 (acidic to basic).
- Application: pH of soil, pH in the human digestive system, and industrial importance of pH in products like shampoos and detergents.
- Types of Questions: Application-based 3-mark or 5-mark questions, often requiring explanation of pH in daily life.
4. Indicators (Litmus, Methyl Orange, Phenolphthalein)
- Key Concepts:
- Litmus: Turns red in acids, blue in bases.
- Phenolphthalein: Colorless in acid, pink in base.
- Methyl Orange: Red in acids, yellow in bases.
- Types of Questions: Practical-based questions, often in Section B, with reasoning on color changes, or 2-mark short answers.

5. Strong and Weak Acids/Bases
- Key Concepts:
- Strong acids/bases: Completely ionize in water (e.g., HCl, NaOH).
- Weak acids/bases: Partially ionize (e.g., CH₃COOH, NH₄OH).
- Types of Questions: 1-mark MCQs or 2-mark questions on comparison, identifying strength, and reasoning.
6. Common Salts and Their Uses (Sodium Chloride, Baking Soda, Washing Soda, Plaster of Paris)
- Definitions and Uses:
- Baking soda (NaHCO₃): Used in cooking, fire extinguishers.
- Washing soda (Na₂CO₃·10H₂O): Used in detergents.
- Plaster of Paris (CaSO₄·½H₂O): Used for casts and molds.
- Types of Questions: 3-mark questions on properties, uses, and chemical equations.
7. Neutralization Reaction and Its Applications
- Reaction: Acid + Base → Salt + Water
- Example: H2SO4 + 2NaOH → Na2SO4 + 2H2O
- Applications:
- In Medicine: Antacids to neutralize stomach acids.
- In Agriculture: Liming of soil.
- Types of Questions: 3- or 5-mark questions with real-life application of neutralization reactions.
8. Water of Crystallization
- Key Concept: Water molecules present in salts (e.g., CuSO4⋅5H2O).
- Example: Copper sulfate pentahydrate is blue because of its water of crystallization.
- Types of Questions: 2-mark questions asking for the definition or examples, short answers explaining the role of water in salts.
9. Preparation of Salts
- Key Methods:
- Neutralization Method.
- Direct Combination of Elements.
- Example: HCl + NaOH → NaCl + H2O
- Types of Questions: 3-mark questions asking about methods of salt preparation with examples.
10. Chlor-alkali Process
- Key Reaction: Electrolysis of brine (NaCl) solution.
- Products: Chlorine (Cl₂), Hydrogen (H₂), Sodium Hydroxide (NaOH).
- Types of Questions: 5-mark long answer questions, typically application-based with diagrams on electrolysis.
In the CBSE 2025 board exams, questions will be framed in various formats such as MCQs, competency-based questions, case study-based questions, and assertion-reason questions. Below is a classification of the most important topics from chapter “Acids, Bases, and Salts” of Class 10 Science along with their respective question types.
Important Topics for MCQs
- Definitions of Acids, Bases, and Salts
- Simple definition-based MCQs.
- Example: “Which of the following is an acid? (a) NaOH (b) HCl (c) NH₄OH (d) Ca(OH)₂”
- Indicators and Their Color Changes (Litmus, Phenolphthalein, Methyl Orange)
- Color changes in acidic and basic media.
- Example: “Phenolphthalein turns which color in a basic solution?”
- pH Scale and Its Ranges
- Identify pH ranges for acidic, neutral, and basic solutions.
- Example: “A solution has a pH of 3. It is: (a) acidic (b) basic (c) neutral (d) none”
- Water of Crystallization
- Example: “Which of the following contains water of crystallization? (a) NaCl (b) CuSO₄·5H₂O (c) Na₂CO₃ (d) KOH”
- Strong vs Weak Acids/Bases
- Identifying strong or weak acids/bases.
- Example: “Which of the following is a weak acid? (a) HCl (b) H₂SO₄ (c) CH₃COOH (d) NaOH
Important Topics for Competency-Based Questions
- Chemical Properties of Acids and Bases
- Predicting reaction products based on given situations.
- Example: “Given a reaction between an acid and a metal, explain the products formed and identify the gas released.”
- pH and Its Importance in Real-Life Situations
- Application-based questions related to daily life.
- Example: “A farmer wants to reduce the acidity of the soil. What will he add to the soil and why?”
- Neutralization Reaction and Everyday Applications
- Practical applications such as antacids, toothpastes, or lime for soil neutralization.
- Example: “Explain how antacids work to relieve acidity in the stomach using the concept of neutralization.”
Important Topics for Case Study-Based Questions
- Chlor-Alkali Process
- Electrolysis of brine solution with an industrial context.
- Example: “A case study presents a factory using electrolysis of brine. Identify the products formed and their industrial uses.”
- Preparation of Salts
- Methods of salt formation and applications in different industries.
- Example: “A company wants to produce sodium chloride through neutralization. What reactions should they use, and what by-products will be formed?”
- Common Salts and Their Uses
- Focus on salts like baking soda, washing soda, and plaster of Paris.
- Example: “Given a scenario about the use of washing soda in cleaning, explain its chemical composition and how it helps in removing hard water stains.”
Important Topics for Assertion-Reason Questions
- pH Scale and Strength of Acids/Bases
- Example: “Assertion: Lemon juice has a pH less than 7. Reason: Lemon juice is acidic. (a) Both assertion and reason are correct (b) Assertion is correct, but reason is wrong.”
- Strong and Weak Acids/Bases
- Example: “Assertion: HCl is a strong acid. Reason: It completely ionizes in water. (a) Both assertion and reason are true (b) Assertion is true, but reason is false.”
- Acid-Metal Reactions
- Example: “Assertion: Zinc reacts with dilute hydrochloric acid to produce hydrogen gas. Reason: Zinc is more reactive than hydrogen in the reactivity series. (a) Both assertion and reason are true (b) Assertion is true, reason is false.”
- Water of Crystallization
- Example: “Assertion: Anhydrous copper sulfate is white. Reason: Water of crystallization imparts color to hydrated salts. (a) Both are true (b) Assertion is true, but reason is false.”
Conclusion
Mastering the chapter “Acids, Bases, and Salts” of Class 10 Science requires a thorough understanding of definitions, chemical reactions, and practical applications. For CBSE 2025, focus on key concepts like the pH scale, neutralization reactions, and the preparation of salts, as these topics are frequently tested. Moreover, expect application-based questions related to daily life scenarios, especially regarding pH importance, uses of salts, and neutralization. Lastly, practicing chemical equations and reactions is essential, as they often appear as short-answer or reasoning-based questions.
By preparing strategically for different question types, you can effectively cover all important aspects of the chapter. MCQs will mainly focus on simple definitions, properties, and color changes. Competency-based questions will test understanding through real-life applications, while case study-based questions will require applying concepts to specific scenarios. Assertion-reason questions will test conceptual clarity, often relating two statements to a common scientific principle.
Important Points to Remember:
- Understand chemical equations for acids, bases, and salts.
- Understand the pH scale, its importance, and daily life applications.
- Study the properties of acids, bases, and salts in detail.
- Practice questions related to neutralization.
- Study indicators and color changes carefully for practical questions.
- Be clear with reaction types, especially for competency-based and case-study questions.
- For assertion-reason, always check whether both statements are true and whether they are logically connected.
- Be prepared for reaction-based and application-based questions.
To enhance your preparation even further for CBSE 2025 Exam, you can also connect with us on YouTube, where we will guide you through all these topics in a comprehensive manner. To join us on YouTube, simply click the link below, and you’ve already taken your first step towards scoring better marks.
In the next post, we will discuss the key topics of the chapter ‘Metals and Non-metals’.
Note: All the topics covered in this article are based on the “Acids, Bases & Salts” chapter of Class 10 Science CBSE Board and have been curated after analyzing previous years question papers. This article can be helpful for students aiming for success in exams, but it’s also important to thoroughly understand the entire syllabus and practice regularly.
Best wishes to all students!
Thank you!





please upload important questions for chapter 4.