Class 12 Chemistry CBSE 2025 Exam: 10 Most Important Topics from ‘Solutions’ Chapter
The chapter ‘Solutions’ for CBSE 2025 Exam Class 12 Chemistry is really very crucial. It forms the foundation for understanding the behaviour of solutes and solvents and how different types of solutions behave under various conditions. Over the years, this chapter has consistently carried significant weight in the CBSE Board exams, making it one of the most important chapters to focus on.
Analysing past trends, certain topics have repeatedly been tested, and understanding these topics can help students score higher marks. This guide will list the 10 most important topics from the ‘Solutions’ chapter in the Class 12 CBSE Chemistry syllabus, along with the probable question types for each topic.
Top 10 Most Important Topics for CBSE 2025 Exams
1. Raoult’s Law
-
- Definition: Raoult’s Law states that the partial vapor pressure of each volatile component in a solution is directly proportional to its mole fraction.
- Formula: PA=XA⋅P0A
- Questions Expected: Numerical questions involving vapor pressure of solutions; theoretical questions on deviations from Raoult’s Law (Positive or Negative Deviation).
- Question Type: Numerical (3 or 5 marks), Short Answer (2 or 3 marks)
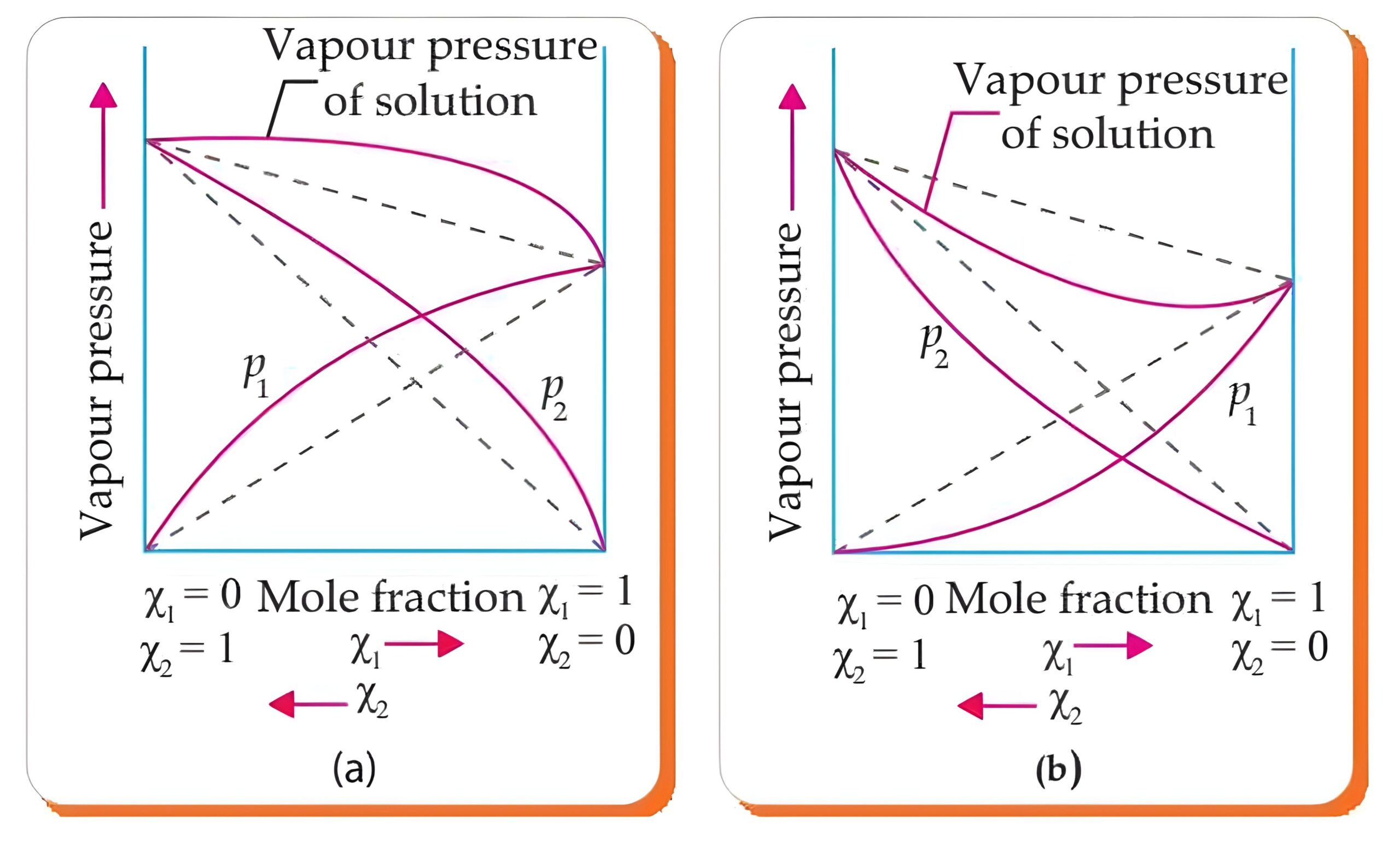
2. Ideal and Non-Ideal Solutions
-
-
- Definition: Ideal solutions follow Raoult’s Law across the entire range of concentrations, while non-ideal solutions deviate from Raoult’s Law.
- Concepts: Positive and negative deviations.
- Questions Expected: Definitions and explanation with examples; comparison between ideal and non-ideal solutions.
- Question Type: Short Answer (2 or 3 marks), Theory-based questions (5 marks)
-
3. Colligative Properties and their Applications
-
- Definition: Colligative properties depend on the number of solute particles and not their identity. Includes relative lowering of vapor pressure, elevation of boiling point, depression of freezing point, and osmotic pressure.
- Formula:
- For elevation in boiling point: ΔTb = Kb ⋅ m
- For freezing point depression: ΔTf = Kf ⋅ m
- Questions Expected: Numerical problems based on colligative properties and their applications (e.g., determining molar mass, freezing point depression).
- Question Type: Numerical (3 or 5 marks)
4. Henry’s Law
-
- Definition: The solubility of a gas in a liquid is directly proportional to the partial pressure of the gas over the solution.
- Formula: P = kH⋅ X
- Questions Expected: Application-based questions involving solubility of gases in liquids, numericals, and conceptual questions about environmental implications like decompression sickness.
- Question Type: Short Answer (3 marks), Numerical (3 or 5 marks)
5. Molarity, Molality, and Mole Fraction
-
- Definitions:
- Molarity: n/V, where n is the number of moles of solute and V is the volume of the solution in litres.
- Molality: n/W, where n is the number of moles of solute and W is the mass of solvent in kg.
- Mole Fraction: Mole Fraction = Moles of solute or solvent/Total moles in solution
- Questions Expected: Simple numerical problems to calculate molarity, molality, and mole fraction; conversion questions.
- Question Type: Numerical (2 or 3 marks), Short Answer (2 marks)
- Definitions:
6. Abnormal Molar Mass and Van’t Hoff Factor
-
- Definition: Abnormal molar mass occurs due to dissociation or association of solute particles. Van’t Hoff factor (i) accounts for this in colligative properties.
- Formula: i=Normal molar mass/Observed molar mass
- Questions Expected: Conceptual and numerical questions, particularly about calculating molar mass in solutions with dissociation or association.
- Question Type: Numerical (3 or 5 marks), Short Answer (2 marks)
7. Osmotic Pressure and Its Applications
-
- Definition: The osmotic pressure of a solution is the pressure that needs to be applied to prevent the inward flow of water across a semipermeable membrane.
- Formula: π = iCRT
- Questions Expected: Application-based questions such as determining molecular mass using osmotic pressure, and real-life applications.
- Question Type: Numerical (3 or 5 marks), Theory-based Short Answer (3 marks)
8. Elevation of Boiling Point
-
- Definition: The elevation in the boiling point of a solution occurs when a non-volatile solute is added to a solvent.
- Formula: ΔTb = Kb ⋅ m
- Questions Expected: Numerical problems on boiling point elevation, particularly calculating the molar mass of solutes.
- Question Type: Numerical (3 or 5 marks)
9. Depression of Freezing Point
-
- Definition: The depression in freezing point occurs when a non-volatile solute lowers the freezing point of a solvent.
- Formula: ΔTf = Kf ⋅ m
- Questions Expected: Numerical problems on freezing point depression; conceptual questions.
- Question Type: Numerical (3 or 5 marks)
10. Solubility and Factors Affecting Solubility
-
- Definition: Solubility refers to the maximum amount of solute that can dissolve in a solvent at a given temperature.
- Factors: Temperature, nature of solute and solvent, pressure (for gases).
- Questions Expected: Short theory-based questions on how temperature and pressure affect solubility.
- Question Type: Short Answer (2 or 3 marks)
Conclusion
Understanding these core topics from the ‘Solutions’ chapter can significantly boost your performance in the Class 12 Chemistry board exam. Each topic has high chances of being asked either as a numerical problem or a theoretical question. Make sure to practice both conceptual understanding and numerical applications, as both are crucial for securing full marks.
To enhance your preparation even further for CBSE 2025 Exam, you can also connect with us on YouTube, where we will guide you through all these topics in a comprehensive manner. To join us on YouTube, simply click the link below, and you’ve already taken your first step towards scoring better marks.
In the next post, we will discuss the key topics of the chapter ‘Electrochemistry.’
Thank you!
Note: All the topics covered in this article are based on the “Solutions” chapter of Class 12 CBSE Board Chemistry and have been curated after analyzing previous years question papers. This article can be helpful for students aiming for success in exams, but it’s also important to thoroughly understand the entire syllabus and practice regularly. Best wishes to all students!
Class 12 Chemistry CBSE 2025 Exam: Important Topics for Objective, Competency-Based, Case Study, and Assertion-Reason Type Questions Click Here